Background: Acute myeloid leukemia (AML) therapy in older patients (age ≥ 60) has undergone a transformation with the introduction of multiple targeted therapies, which has allowed more patients to receive therapy than before. The most successful has been the combination of venetoclax + azacitidine (VA) which generated higher overall complete response rates in phase 2 studies than would be expected with azacitidine alone, leading to accelerated FDA approval on 21Nov2018. The VIALE-A phase 3 study confirmed the improved overall survival (OS, median 14.7 versus 9.6 months) of VA, leading to full FDA approval for marketing. This retrospective analysis provides real world data on the impact of baseline clinical and genomic markers of individuals receiving intensive chemotherapy versus VA.
Methods: The precision medicine Beat AML Master Trial (NCT03013998) assigns patients to biomarker specific sub-study treatments based on targeted DNA sequencing and cytogenetics. However, a large subset of patients did not enroll on a sub-study, but were followed for off-study treatment and OS. From this subset, patients enrolled onward from 21Nov2018, when VA received accelerated approval, were examined for differences in clinical/genetic characteristics and OS in those receiving venetoclax + hypomethylating agent (V/HMA), any form of intensive chemotherapy (IC), alternative non-intensive therapy (NIT), or no therapy. The genetic mutations identified in this study are characterized by a variant allele frequency of 20%+, as defined in the trial for determining the dominant clone. The method of Kaplan-Meier was used to estimate OS, and the Cox model was fit to associate patient characteristics with OS.
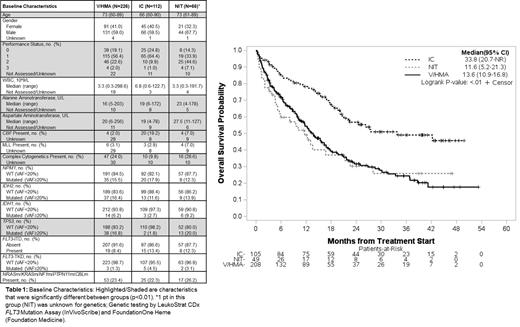
Results: From 21Nov2018, a total of 468 AML patients consented to the Beat AML trial and did not enroll to a sub-study. Treatment was chosen by the investigator based upon available clinical and genomic data. Of these 468 patients, 62 did not receive treatment, 2 had treatment data missing, 226 were treated with V/HMA, 112 with IC, and 66 with NIT. Demographics for all patients include median age 71 (60-90), 40% female, performance status (PS) (0-20%; 1-55%; 2-22%; 3-3%), median white blood cell (WBC) 4.1 (range 0.3-298.6), complex karyotype (CK) 20.6%, core binding factor (CBF) 7.8%, KMT2A-rearranged 3.6%, NPM1-mutated (m) 15.6%, IDH2m 14.6%, TP53m 13.2%, FLT3-ITD/TKD 12.9%, and NRASm/ PTPN11m/ KRASm/ NF1m/ CBLm 23.6%. Demographics that were significantly different (p<0.01) among the three groups (V/HMA, IC, and NIT) include age (younger in IC), PS (worse in NIT), AST/ALT (worse in NIT), CBF (more in IC), CK and TP53m (less in IC). Of the 404 patients who underwent treatment with V/HMA, IC, or NIT, 208 have died with those surviving having a median follow-up of 22.3 months. Figure 1 summarizes the OS of each treatment group. The median OS (95% CI) from time of initiating therapy is 13.6 (10.9-16.8) for V/HMA, 33.8 (20.7-not reached) for IC, and 11.6 (5.2-21.3) months for NIT. Univariable analysis for OS was significant at p<0.05 for increased age, WBC, PS, hemoglobin, CBF, CK, NPM1m, TP53m, TET2m and IC versus V/HMA. Multivariable analysis was significant at p<0.05 (hazard ratio) for increased age (1.14), PS (1.83), WBC (1.08), hemoglobin (0.91), and select genomic aberrations including CBF (0.37), NPM1m (0.35), and TP53m (2.1).
Conclusions: These results from a large cohort of older AML patients treated with V/HMA, IC, or NIT show that their outcome is best defined by pre-treatment clinical features previously identified including age, performance status, WBC and hemoglobin along with limited genomic characteristics including CBF, NPM1m, and TP53m. While univariate analysis of OS favored IC over V/HMA and NIT, multivariable analysis supports that this advantage was most likely due to the favorable clinical and genomic features. The OS of V/HMA patients in this cohort is similar to that in the VIALE-A registration study of VA, providing further justification for this treatment for older AML patients deemed ineligible for intensive chemotherapy. Additionally, this data supports use of the patient cohort in this study for ongoing work in understanding and/or validating biomarkers associated with survival with V/HMA.
Disclosures
Borate:Servier: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Pfizer: Other: Research; Astellas: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; RUNX1 Foundation: Honoraria; Jazz: Other: Research; Incyte: Other; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Research. Swords:Kronos Bio: Research Funding. Traer:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Schrodinger: Research Funding; Astra-Zeneca: Research Funding; Incyte: Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees; Prelude Therapeutics: Research Funding. Stein:Aptose: Consultancy; PinotBio: Consultancy; Menarini: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Genesis: Consultancy; Jazz: Consultancy; OnCusp: Consultancy; Astellas: Consultancy; Novartis: Consultancy; Agios: Consultancy; Syros: Consultancy; Abbvie: Consultancy; Gilead: Consultancy; Neoleukin: Consultancy; Eisai: Research Funding; Syndax: Consultancy; Bristol Myers Squib: Consultancy, Research Funding; CTI Biopharma: Consultancy; Foghorn: Consultancy; Daiichi: Consultancy; Calithera: Consultancy; Servier: Consultancy; Ono Pharma: Consultancy; Blueprint: Consultancy. Lin:Bio-path Holdings: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Celyad: Research Funding; Aptevo Therapeutics: Research Funding; Cleave Biosciences: Research Funding; Ciclomed: Research Funding; Jazz Pharmaceuticals: Research Funding. Madanat:OncLive: Honoraria; Novartis: Honoraria; Taiho oncology: Honoraria; Stemline therapeutics: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Sierra Oncology: Honoraria; GERON: Consultancy; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement; MD Education: Honoraria; Rigel Pharmaceuticals: Honoraria. Patel:Servier LLC: Current Employment. Baer:Abbvie (Inst): Research Funding; Takeda (Inst): Research Funding; Kura Oncology (Inst): Research Funding; FORMA Therapeutics (Inst): Research Funding; Kite, a Gilead company (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding. Stock:Newave: Honoraria; Kite: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria; Servier: Other: Data Safety Monitoring Board/Advisory Board; Kura: Research Funding; Glaxo Smith Kline: Consultancy; Amgen: Honoraria. Odenike:ABBVIE, Astrazeneca, Agios, Aprea, Astex, BMS/Celgene, CTI, Daiichi, Incyte, Janssen, Kartos, Novartis, NS-Pharma and Oncotherapy Sciences: Research Funding; BMS/Celgene, Novartis, Rigel, Servier, Taiho ; DSMB-Kymera therapeutics: Membership on an entity's Board of Directors or advisory committees. Kovacsovics:Servier: Consultancy; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Gilead: Research Funding; Novartis: Research Funding; Syndax Pharmaceuticals: Research Funding. Deininger:Novartis, Takeda, Blueprint, Incyte, Dava Oncology, CTIBio, Syneos, Cogent, Pfizer, Dispersol: Consultancy. Zeidner:Daiichi Sankyo: Honoraria; Foghorn: Consultancy; Gilead: Consultancy, Honoraria, Research Funding; Immunogen: Honoraria; Jazz: Research Funding; Merck: Research Funding; Novartis: Consultancy; Sellas: Consultancy; Servier: Consultancy, Honoraria; Shattuck Labs: Honoraria, Research Funding; Stemline: Research Funding; Sumitomo Dainippon Pharma: Research Funding; Takeda: Research Funding; Astex: Research Funding; Arog: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Olin:Cellectis: Research Funding; Servier: Consultancy; Abbvie: Consultancy; Rigel: Consultancy; Astellas: Consultancy; Actinium: Consultancy. Smith:Cellgene: Other: Clinical trial funding; Abbvie: Honoraria, Research Funding; Genentech: Honoraria; ERASCA: Research Funding; Revolution Medicines: Research Funding; Zentalis: Other: Clinical trial funding. Foran:BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Actinium: Research Funding; Kura: Research Funding; Sellas: Research Funding; Roivant: Research Funding; Novartis: Research Funding; Celgene: Research Funding; Astellas: Research Funding; NCI: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees. Schiller:Karyopharm Therapeutics: Research Funding, Speakers Bureau; Sanofi: Research Funding, Speakers Bureau; Astellas Pharma: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding; Incyte: Consultancy, Research Funding, Speakers Bureau; Kite: Research Funding, Speakers Bureau; Agios: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Actinium Pharmaceuticals: Research Funding; Stemline Therapeutics: Speakers Bureau; Novartis: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding, Speakers Bureau; Agios: Consultancy; Constellation Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding; Celator: Research Funding; Arog: Research Funding; Actuate Therapeutics: Research Funding; Fujifilm: Research Funding; FORMA Therapeutics: Research Funding; Delta-Fly Pharma: Research Funding; Deciphera: Research Funding; Genentech/Roche: Research Funding; Gamida Cell: Research Funding; Onconova Therapeutics: Research Funding; Mateon Therapeutics: Research Funding; Geron: Research Funding; Precog: Research Funding; Pfizer: Research Funding; Sangamo Bioscience: Research Funding; Samus Therapeutics: Research Funding; REGiMMUNE: Research Funding; Stemline Therapeutics: Research Funding; Sellas Life Sciences: Research Funding; Tolero Pharmaceuticals: Research Funding; Takeda: Research Funding; ElevateBio: Research Funding; Trovagene: Research Funding; Ono Pharmaceutical: Research Funding; AVM Biotechnology: Research Funding; Syros Pharmaceuticals: Research Funding; Kronos Bio: Research Funding; Ono Pharmaceutical: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Amgen: Current equity holder in publicly-traded company, Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Research Funding, Speakers Bureau. Curran:Amgen: Other: Advisory board; Servier: Consultancy, Other: Expert consensus panel; Jazz: Other: Advisory board; Pfizer: Honoraria, Other: Advisory board; Incyte: Other: Advisory board; Kite: Other: Advisory board. Druker:Astellas: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Gilead: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; US Patent and Trademark Office: Patents & Royalties: Patents 6958335 (Novartis exclusive license), 4326534, 7416873, 7592142, 10473667, 10664967, 11049247; Syndax: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Incyte: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Tolero: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Vincerx Pharma, Inc.: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; VB Therapeutics: Membership on an entity's Board of Directors or advisory committees; The RUNX1 Research Foundation: Membership on an entity's Board of Directors or advisory committees; Recludix Pharma, Inc.: Consultancy, Current holder of stock options in a privately-held company; Labcorp: Membership on an entity's Board of Directors or advisory committees; Nemucore Medical Innovations, Inc.: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Beat AML LLC: Membership on an entity's Board of Directors or advisory committees; Multicancer Early Detection (MCED) Consortium: Membership on an entity's Board of Directors or advisory committees; Aileron Therapeutics: Membership on an entity's Board of Directors or advisory committees; Therapy Architects, LLC: Membership on an entity's Board of Directors or advisory committees; CureOne: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: PI or Co-investigator on clinical trial(s) funded via contract with OHSU., Research Funding; AstraZeneca: Other: PI or Co-investigator on clinical trial(s) funded via contract with OHSU.; Enliven Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oregon Health & Science University: Current Employment, Patents & Royalties: #1518 (exclusive option agreement with CytoImage); #0606/Patent 6958335 (Novartis exclusive license); #2573; #0843; #0996; DNA SEQ: Membership on an entity's Board of Directors or advisory committees; Dana-Farber Cancer Institute: Patents & Royalties: #2063 (licensed exclusively to Merck & Co); and #2524, Research Funding; Bristol Myers Squibb: Other: Co-investigator on clinical trial(s) funded via contract with OHSU.; Iterion Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; GRAIL: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Cepheid: Membership on an entity's Board of Directors or advisory committees; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Aptose Biosciences: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Adela, Inc.: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Amgen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Levine:Ajax: Membership on an entity's Board of Directors or advisory committees, Research Funding; Zentalis: Membership on an entity's Board of Directors or advisory committees, Research Funding; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Roche: Honoraria; Prelude: Membership on an entity's Board of Directors or advisory committees; Auron: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Lilly: Honoraria; Janssen: Consultancy; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Qiagen: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria. Burd:Eilean Theraputics: Current Employment, Current equity holder in private company. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Byrd:Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal